Dr David Guex
March 2015 - Le Fil Dentaire
Download the PDF
There is nothing more frustrating in endodontics than the cleaning and disinfection protocols, and finally having a tooth that does not react to our treatment.an asymptomatic tooth becomes painful, or the pathology does not disappear, etc. Why, what about it, why it did not worked out?
These are the articles that will help us understand
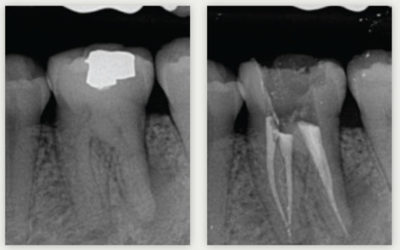
First of all we must take into account the complexity of the anatomy of the root canal system. This was described by Dr. Walter Hess, the famous plates of Hess[1]. These drawings made out if chinese ink in 1917 show all the complexity of the root canal system: isthmus, handles, lateral canals... By seeing his drawings every Dental Surgeon understand the interest of keeping teeth alive, but sometimes this is not possible.
When we need to perform biopulpectomy or retreatment, the surgical microscope provides us with a lot of help. We work with the spine straight, see our canal orifices because they are illuminated by the light of the microscope and because its optics grow big these orifices. Canal orifices not visible to the human eye become reachable with tools. The most important example is the "fourth canal" of the first maxillary molar. This fourth canal is present in almost 100% of the cases.
In correlation with Hess's plates, we can link the Peters[2] article: "on the maxillary molars, whatever the technique used, more than 35% of the ductal surfaces remain un-instrumented." it is enormous. But what we definitly must integrate on anatomy are not only the canals and their branches, but especially the opinion that Scanning Electron Microscopy carries on the canal walls: the dentinal tubuli. Their number is gigantic.
Dental tubules have enough space to welcome bacteria[3]. The anatomical challenge becomes major.
But what happens when the anatomy is contaminated with bacteria?
In a first step, the bacteria progress by DIVISION rather than by displacement[4]. This means that in order to reach the apex, their number must increase. We find at the apex these are the most pathogenic bacteria[5]. Because of course, when we give them time, the bacteria will organize themselves into a biofilm. A biofilm consists of about 15% bacteria and 85% matrix[6]. The endodontic flora contains more than 500 species[7], and this biofilm becomes 1000 to 2000 times more resistant to antiseptic solutions[7bis].for example the necessary concentration of antibiotic to kill bacterial strains in a biofilm is 250 times greater than for bacterial strains with planktonic growth[8].
Thus, the conjunction between endodontic anatomy is the degree of bacterial contamination shows that we will have to irrigation energy AND for destructuring the bacterial biofilm AND for diffusing our products into the dentinal tubules. The disinfecting solution of choice is sodium hypochlorite having a concentration between 2.5 and 6%. It has a broad spectrum against bacteria, spores, yeasts, viruses[9], and it dissolves tissues thanks to its ability to oxidize and hydrolyze cellular proteins[10].
The first means of diffusing sodium hypochlorite into peri-apex is the instrumentation of the canal.this allows us to create a space to transport the irrigant and to cut the contaminated dentine[11].
However, by instrumenting a channel, we create an endogenous substance: the smear layer. It contains organic and inorganic substances composed of micro-organisms and necrotic debris[12]. This layer of smear layer may be packed from the inside of the dentinal tubules up to 40 µm deep[13]. The smear layer may be infected and can protect the bacteria present inside the dentinal tubules by preventing effective action of duct disinfectants[14]-[15].
Therefore we must use the DTA at 17% in order to dissolve the mineral part thanks to its chelating action[16].
Already in 1943, Grossman defined a specification on the general principles of endodontic irrigation[17]. For an efficient irrigation, the used solutions have to allow a canal "wash" by an antiseptic action, by eliminating microorganisms, by eliminating dentinal chips, by allowing a file lubrification, not being agressive for the peri-apex.
However, all studies clearly show that it is not possible to sterilize an infected root canal system because the apical area represents the limit of irrigation efficiency[18]-[19]. O'Connell et al. in 2004 (quoted by Gu) show that irrigation is only effective in the first two thirds of the canal. And two studies show that even if a needle is brought to LT-1, the smear layer remains at the apical level[20]. So we understand that despite our efforts to irrigate a canal, irrigants can not always go to the apex.the reason is simple: the presence of the air column in the canal[21]. This air column prevents the irrigant from going further than the tip of the irrigation needle[22].
Thus, in order to increase the efficiency of the irrigation solutions and their surface of action, an activation of these irrigation solutions[23] is recommended.
The first activation technique, one of the most effective and cheapest, is dynamic manual agitation. It consists in using a cone of gutta percha adjusted to the apical foramen, and to perform vertical movements of back and forth. These cause a hydrodynamic effect which greatly improves the displacement and therefore the efficiency of the irrigation solutions[24].
The second most widely used activation technique in dental surgeries and the use of ultrasound. Ultrasound is a vibratory energy generating acoustic waves with fluid motion[25]. But it is important to note that if the file comes into contact with the canal wall father, this cavitation phenomenon is canceled, since the waves are lost in the dentin walls[26]. However, ultrasonic studies are contradictory.it emerges that ultrasound is effective in the first two thirds of the radicle[27].
So how do we go beyond the limits of these activation methods? It is necessary to transmit to the irrigation solutions an energy which favors its global agitation by diffusing in the irrigators with the least possible constraints.
Thinking of ENERGY, we can think of lasers. A laser has the particularity of emitting an intense light wave in the very determined direction, frequency and manner. It is a so-called coherent light, contrary to, for example, that emitted by a filament bulb which emits numerous zones of frequencies and various phases and this in all directions.
Hirono Takeda in 1998 showed that in endodontics, the most efficient laser is the Erbium:YAG laser for the removal of debris and the smear layer[28]. Because this column of air that keeps us from spreading irrigants and will become an asset for the Erbium:YAG laser. Activation of the erbium laser in an aqueous environment generates large bubbles that magnify and then explode. This expansion causes a high pressure in the fluids[29].
The use of the Erbium:YAG laser on 6.25% sodium hypochlorite shows a bacterial reduction close to 100% on teeth contaminated with Enterococcus faecalis[30], whether on canal walls or tubuli 100 to 200 μm[30bis].
Moreover, whatever the technique used (irrigation syringe, ultrasound), the use of Erbium:YAG significantly increases the cleaning of the infected dentin[31].When we look under a microscope a laser tips Er:YAG, in a static position, located 2 or 3 millimeters from the entrance of the mesio lingual canal of the first mandibular molar, and as soon as we activate the irrigation solutions, it is frequent to observe debris coming out of the mesio vestibular canal; even though the two canals are anatomically independent. We can deduce that the energy diffused in the liquids is so deep inside that the debris have been pushed out of the isthmus connecting the mesio lingual and mesio vestibular canal.
We have a simple way of verifying the veracity of this bibliography; to carry out tests on resin blocks, replicas of resin teeth (True Tooth) or on natural teeth made transparent according to the method of Augusto Malentacca. But regardless of the model used, it will be important that the apex be sealed with adhesive or resin to recreate the sealing conditions of the peri-apex.
In conclusion in our endodontic activity, the combination of the operating microscope with the Erbium:YAG laser represents a technological advance that can help us to be more efficient in our exercise.
Thanks
I would like to thank Dr. Jean-François Sevain for his invaluableband meticulous help in correcting this article, and all the precedents.
This article is the summary of my lecture given at the ADF 2014, I would like to thank the professional and friendly assistance provided by Dr. Jean-Yves Cochet. The very first French Endodontist to have published on the effects brought by laser technology is Dr. D. Bensoussan.
General practitioner in Villié-Morgon (France) since 1999.
Exclusive endodontist in Bron (France) since 2009.
University degree in anatomy and cranio-cervico-facial dissection of the Faculty of Medicine of Paris.
Bibliography
1 The anatomy of the root-canals of the teeth of the permanent dentition, by Walter Hess and the anatomy of the root-canals of the teeth of the deciduous dentition, and of the first permanent molars, by Ernst Zürcher. New York, Wm. Wood & co., 1925.
2 Peters OA, Schönenberger K, Laib A. Effects of four Ni-Ti preparation techniques on root canal geometry assessed by micro computed tomography. Int Endod J 2001 ; 34 : 221-230.
3 Perez F, Calas P, De Falguerolles A, MauretteA, Migration of a Streptococcus sanguis strain through the root dentinal tubules. J Endod 1993 a ; 76 : 97-103.
4 Hall-Stoodley L, Stoodley P. Biofilm formation and dispersal and the transmission of human pathogens, Trend Microbiol 2005 ; 13 : 7-1.
5 Molven O, Olsen I, Kerekes K. Scanning electron microscopy of bacteria in apical part of root canals in permanent teeth with periapical lesions, Endod Dent Traumatol 1991 ; 7 : 226-229.
6 Costertoon W, Veeh R, Shirtliff M, Pasmore M, Post C, Ehrlich G. The application of biofilm science to the study and control of chronic bacterial infections. J Clin Invest 2003 ; 112 : 1466-1477.
7/7bis Svensater G, Bergenholtz G. Biofilms in endodontic infections. Endod Topics.2004 ; 9 : 27-36.
8 Sedlacek MJ, Walker C. Antibiotic resistance in an in vitro subgingival biofilm model. Oral Microbiol Immunol 2007 ; 22 : 333-339.
9 Mc Donnell G, Russel AD. Antiseptic and desinfectants : activity, action and resistance. Clin Microbiol Rev 1999 ; 12: 147-179.
10 Pashley EL, Birdsong NL, Bowman K, Pashley DH. Cytotoxic effects of a NaOCl on vital tissue. J Endod 1985 ; 11 : 525-528.
11 Peters OA. Current challenges and concepts in the preparation of root canal systems : a review. J Endod 30 : 559, 2004.
12 TORABINEJAD M, HANDYSIDES R, KHADEMI A.A., BAKLAND L.K. Clinical implications of the smear layer in endodontics : a review. Oral Surg 2002;94:658-666.
13 MADER C.L., BAUMGARTNER J.C., PETERS D.D. Scanning electron microscopic investigation of the
smeared layer on root canal walls. J Endod 1984;10:477-483.
14 Zehnder M. Root canal irrigants. JOE. 2006 ; 32 : 389-398.
15 HAAPASALO M., ORSTAVIK D. In vitro infection and disinfection of dentinal tubules. J dent Res 1986;66:1375-1379.
16 Shahravan A, Haghdoost AA, Adl A, Rahimi H, Shadifar F . Effects of smear layer on sealingability of a canal obturation : a systematic review of a meta-analysis. J Endod 2007 ; 33 : 96-105.
17 Grossman LI, Meiman BW. Solution of pulp tissue by chemical agents. J Am Dent Assoc. 1941 ; 28 : 223-225.
18 Simon S,Machtou P, Pertot W, Endodontie, édition CdP 2012, collection JPIO, chapitre 11, p 227.
19 Senia ES, Marschall JF, Rosen S. The solvent action of sodium hypochlorite on pulpe tissue of extracted teeth. Oral Surg Oral Med Oral Pathol 1971 ; 31 : 96-103.
20 O’Connell MS, Morgan LA, Beeler WJ, Baumgartner JC. A comparative study of smear layer removal using different salts of EDTA. J Endod 2000;26:739–43.
21 Luks S. Practical endodontics. Philadelphie : JB Lippincott, 1974 : 82-85.
22 Bronnec F, Bouillaguet S, Machtou P, Ex vivo assessment of irrigant penetration and renewal during the cleaning and shaping of root canals : a digital subtraction radiographic study, Int Endod J 2010a ; 43 : 275-282.
23 Zehnder M. Root canal irrigants. JOE. 2006 ; 32 : 389-398.
24 Caron G. Cleaning efficiency of the apical millimeters of curved canals using three different modalities of irrigant activation: an SEM study. Paris VII University, Paris, France: Masters thesis ; 2007.
25 Schäfer E. Irrigation of the root canal. Endo. 2007 ; 1 : 11-27.
26 Van der Sluis L, Versluis M., Wu M, Wesselink P. Passive ultrasonic irrigation of the root canal: a review of the literature. IEJ. 2007 ; 40, 415–426. 415.
27 Mayer BE, Peters OA, Barbakov F,. Effects of rotary instruments and ultrasonic irrigation on debris and smear layer scores : a scanning electron microscopic study . Int Endod J ; 35 : 582, 2002.
28 Takeda FH, Harashima T, Kimura Y, Matsumoto K. Comparative study about the removal of smear layer by three types of laser devices. J of Clinical Laser. 1998 ; 16 : 117-122.
29 Blanken et al (2007) Cavitation as working mechanism of the Er,Cr:YSGG laser in endodontics : a visualization study. Journal of Oral Laser Applications ; 7 : 97-106.
30/30bis Xiaogang Cheng et al. Evaluation of the Bactericidal Effect of Nd:YAG, Er:YAG, Er,Cr:YSGG Laser Radiation, and Antimicrobial Photodynamic Therapy (aPDT) in Experimentally Infected Root Canals.. Lasers in Surgery and Medicine 44:824–831 (2012).
31 Ordinola-Zapata and al. Biofilm removal by 6 % sodium hypochlorite activated by different irrigation techniques. Int Endod J ; nov 2013.