Dr David Guex
February 2014 - Le Fil Dentaire n°90
Download the PDF
One of the challenges of endodontics is to destroy as much as possible the bacterial load. One of the means used is the irrigation, which has been attempted to be activated by different techniques. Today, the miniaturization of the tips and the maneuverability of the Erbium:YAG laser handpiece allow the channel to access the blind spots.
The bacterial biofilm
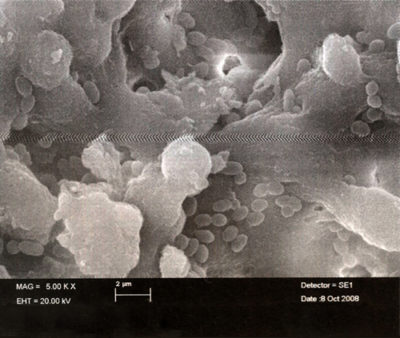
The penetration of bacteria into the root canal system marks the beginning of problems.
- At first, the contamination is caused by planktonic bacteria [1]-[2] which are fairly easy to destroy (Fig. 1), but they quickly organize into a biofilm.
- Inside of the biofilm a competition is organized between the bacteria to finally find the aerobies on the surface, as agent of protection of the anareobies located inside.
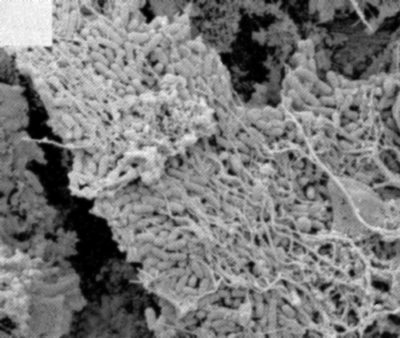
As a result, 250 times more antibiotics are needed to destroy the bacteria biofilm than the planktonic bacteria.on the other hand, the bacteria migrate in the root canal system not by displacement but by multiplication (Fig.3) [3]-[4]. This means that the amount of bacteria increases exponentially with time.
It seems that each tooth has a unique bacterial infection model in which the presence of a bacterial biofilm is the rule.
The bacteria of the endodontic flora colonize the entire root canal system, so we find them:
- on remains of necrotic pulp tissue where it finds the necessary nutrients for their growth
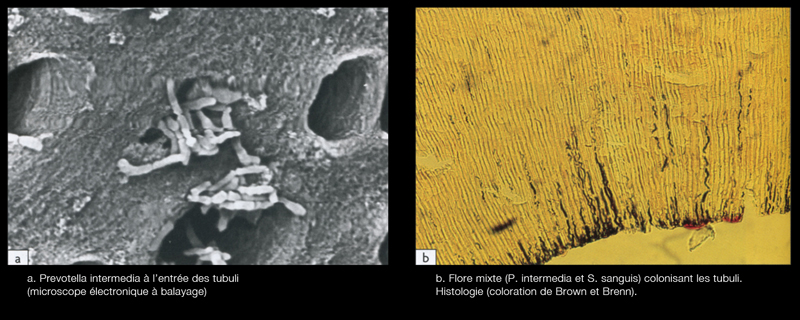
- along the dentinal walls where they stick to one another on several layers, organized in biofilm (Fig.5)
- in accessory canals and apical deltas
- inside the dentinal tubules at a depth of several hundred microns, the bacteria are preferentially housed in the first third of the peri-canal dentine (Fig.6)[5]

However, it is illusory to attempt to "sterilize" the infected canal, a complete elimination of bacteria is not feasible, but our therapeutic maneuvers must aim at a significant reduction of the ductal bacterial load in order to achieve an acceptable "critical rate" which guarantees the success of endodontic treatment[6].
Endo-canal anatomy
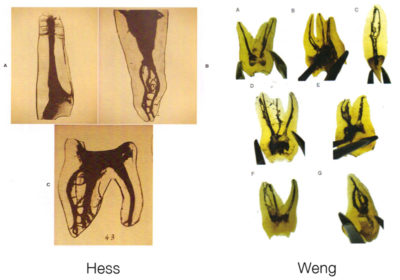
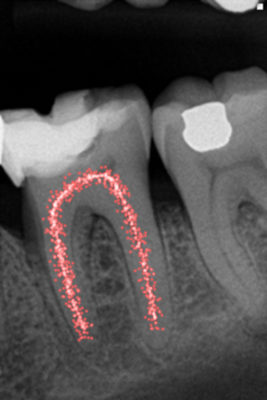
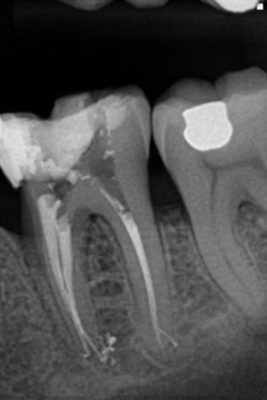
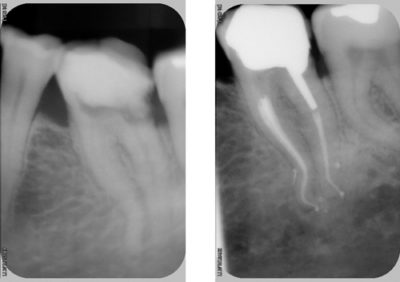
Weng's work in 2009 only confirms what Hess had before him in 1917, namely that pulpar anatomy is infinitely more complicated than a simple canal in his root (Fig. 7). It is more meandering, anastomosis and labyrinths.
Means of chemical disinfection
There are 2:
Sodium hypochlorite (concentrated at 5 - 6%): it has a broad antibacterial spectrum (bacteria, spores, yeasts, viruses). This action is due to its capacity for oxidation and hydrolysis of cellular proteins. Its hypertonicity permits by diffusion the evacuation of the cellular fluids, the solution must arrive at the contact of the tissues[7].
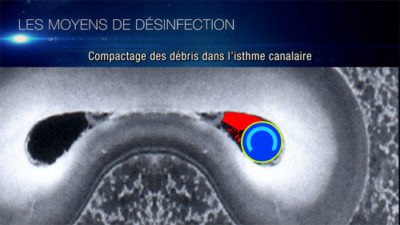
Craig Barrington shows the contamination of this isthmus (Fig. 8) we understand the importance of diffusion of the irrigation solution in order to eliminate the bacterial biofilm beyond the main canals.
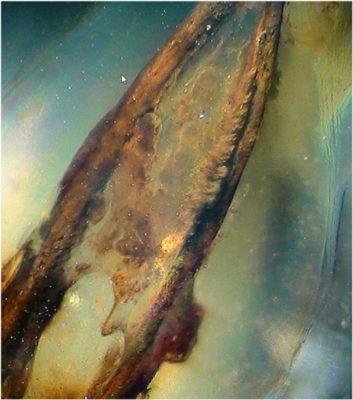
EDTA at 17%: this liquid allows the elimination of the smear layer. The instrumental maneuvers carried out during root canalization create a film of smear layer of 1 to 5 μm thick, composed of an aggregate of organic and debris (Fig. 9). Its elimination allows a complete ducting stop as well as the sealing of its obturation[8].
Mechanical disinfection means
Shaping: its purpose is to take irrigant to the apex and eliminate the contaminated dentin.
However, on maxillary molars, regardless of the rotary technique used, more than 35% of the root canal surfaces remain uninstrumented [9]-[10].
The irrigation syringe
All the recent studies concluded that the apical third represents the limit of efficiency of irrigation[11]. Yet on the infected teeth, it is in the last apical mm that the nocive bacteria are located[12].
The irrigation solution delivered to the syringe without wedging the needle into the channel does not go further than the tip of the needle due to the presence of an air column[13] surprisingly, we rediscover its importance today[14] (Fig. 11).
Fig. 11: Apical air bubble
The penetration and the exchange of the solution are improved with the progress of the shaping.
Irrigation is only really effective at the end of the shaping. The reflux space between the needle of the syringe and the dentinal walls allows the creation of a hydraulic circuit which allows a progressive exchange of the solution.
The ultrasound
The major effect of the US and especially the rise in temperature generated on the hypochlorite which increases its antibacterial power. On the other hand, its diffusion effect in the different areas of the root canal system is more restricted than if the US touches the canal walls, this recreates a layer of smear layer that will have to be removed again.
The Erbium:YAG laser
Operation
Atomic ionization creates a plasma (Fig. 13). This plasma creates an increase in pressure (Fig. 14).
Fig. 13: Blast (Courtesy Syneron)
Fig. 14 (Courtesy Syneron)
At the same time there is an explosion of the water molecule (Fig. 15).
Fig. 15 (Courtesy Syneron)
The increase in pressure coupled with the explosion of the water molecule generates a shock wave called BLAST.
This blast causes both a bursting of the bacterial membranes and a better diffusion of the irrigants in the root canal anatomy.
The amplification of the photoacoustic effect induced by a static laser fiber located 2 mm above the root canal entry improves the agitation of the irrigation solutions and therefore increases their circulation in the root canal network (Fig. 12). It is necessary to shoot into the present solution in the pulp chamber.
From the reading of article 1 of the bibliography, the best way to reduce the bacterial population in dentinal tubules to a depth of 100 to 200 ym from the dentin walls is the use of coupled Erbium:YAG to hypochlorite (Fig. 16).
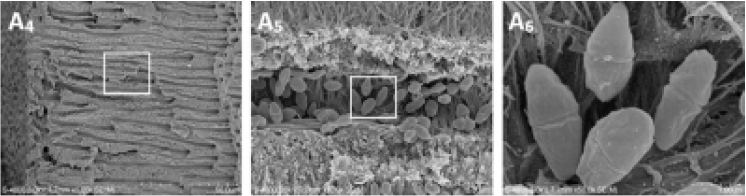
Also from article 1 on teeth artificially contaminated with enterococcus faecalis, the Erbium:YAG laser working in distilled water eliminates this bacteria on 99.97% of the dentin walls. This indicates that the agitation caused by Erbium:YAG is highly effective (Fig. 17).
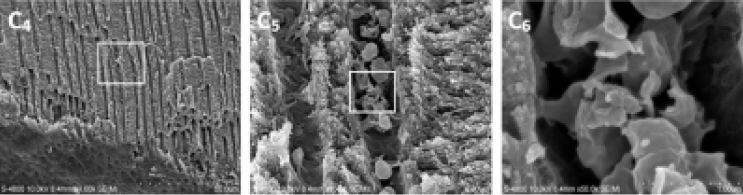
On the other hand, the study by Ordinola-zapata and coll[15] shows that on teeth artificially contaminated by 4 types of bacterial biofilm removal efficiency is significantly increased by using the Erbium:YAG laser in sodium hypochlorite.
Erbium:YAG activation of sodium hypochlorite for 0.0842 seconds (= 1684 shots of 50 μs) corresponds to the Ultrasounds + Self Adjusting File combined operation for 2 minutes[16].
This means that if we activate the Erbium:YAG laser hypochlorite: 12 seconds per channel and if we activate the EDTA: 6 / seconds per canal this makes a total of 18 seconds per canal, so for a molar consisting of four canals this corresponds to 72 seconds.
72 seconds of laser activity Erbium:YAG corresponds to a US + SAF cleaning of this same molar of 3 hours and a half.
We have carried out work on transparent teeth, which are then pressed back into transparent resin so that the apexes are tight. Our primary objective is to recreate the weight of the air column in the canal.
Our findings are as follows:
- when we activate laser shots in a NO prepared canal (eg distal root of a 16) the other unprocessed canals are also activated (palatine root and MB1),
- very often the accessory canals are cleaned before the apical part.
In vivo, whatever system used to potentiate the irrigant, we always see a rise in additional debris when the Erbium:YAG laser is used.
The most spectacular effect is the rise of a large package of smear layer aggregates into a canal while the laser tips works in another canal.
Conclusion
Canal cleaning depends on the relationship between two interrelated factors: the anatomy of the root canal system and the bacteria.
To this date it seems to me that the Erbium:YAG laser brings new solutions:
- A larger rise in organic debris than the irrigation syringe.

Irrigation syringe (c) Carlos Matias - A greater rise of smear layer than the irrigation syringe.
The more complicated the endodontic, with isthmus, meanders, anastomoses, the more the bacteria have time and the more they organize themselves in biofilm: it will require even more energy to destroy them.
Hence this concept of cleaning energy dependent canal that we propose.
General practitioner in Villié-Morgon (France) since 1999.
Exclusive endodontist in Bron (France) since 2009.
University degree in anatomy and cranio-cervico-facial dissection of the Faculty of Medicine of Paris.
Bibliography
1 XIAOGANG CHENG AND AL. Evaluation of the Bactericidal Effect of Nd:YAG,Er:YAG, Er,Cr:YSGG Laser Radiation, and Antimicrobial Photodynamic Therapy (aPDT) in Experimentally Infected Root Canals Lasers in Surgery and Medicine ; 44 : 824–831 (2012)
2 F. PAQUÉ, C. BOESSLER & M. ZEHNDER Accumulated hard tissue debris levels in mesial roots of mandibular molars after sequential irrigation steps Volume 44 number 2 February 2011 page 148- International Journal of Endo
3 PEREZ F, CALAS P, DE FALGUEROLLES A, MAURETTE A. Migration of a Streptococcus sanguis strain though the root dentinal tubules J Endod 1993a ; 19 : 297-301
4 PEREZ F, CALAS P, ROCHD T effect of dentin treatment on in vitro root tubule bacterial invasion Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1996 ; 82 : 446-451
5 PEREZ F Endodontie 2012 CdP, chapitre 7, p 126
6 PEREZ F Endodontie 2012 CdP, chapitre 7, p 131
7 PASHLEY EL, BIRDSONG NL, BOWMAN K, PASHLEY DH. Cytotoxic effects of NaOCL on vital tissue J Endod 1985 ; 11 : 525-528
8 MADER CL, BAUMGARTNER JC, PETERS DD. Scanning electron microscopic investigation of the smeared layer on root canal walls J Endod 1984 ; 10 : 477-483
9 BERGMANS L, VAN CLEYNENBREUGEL J, WEVERS M, LAMBRECHTS P. A methodology for quantitative evaluation of root canal instrumentation using microcomputed tomography Int endod J 2001; 34 : 390-398
10 PETERS OA, BOESSLER C, PAQUÉ F Root canal preparation with a novel nickel-titanium instrument evaluated with micro-computed tomography : cnal surface preparation over time Int endod J 2001 ; 34 : 184-188
11 SENIA ES, MARSHALL JF, ROSEN S. The solvent action of sodium hypochlorite on pulpe tissue of extracted teeth Oral Surg Oral Med Oral Pathol 1971 ; 31 :96-103
12 MOLVEN O, OLSEN I, KEREKES K. Scanning electron microscopy of bacteria in the apical part of roots canal in permanent teeth with periapical lesions Endod Dent Traumatol 1991 ; 7 : 226-229
13 LUKS S. Practical endodontics Philadelphie : JB Lippincott, 1974 : 82-85
14 TAY FR, GU LS, SCHOEFFEL GJ, WIMMER C, SUSIN L, ZHANG K ET AL. Effect of vapor lock on root canal debridement by using a sidevented needle for positivepressure irrigant delivery J endod 2010 ; 36 : 745-750
15 ORDINOLA-ZAPATA ET COLL. Biofilm removal by 6 % sodium hypochlorite activated by different irrigation techniques International Endodontic Journal november 2013
16 SEDLACEK MJ, WALKER C. Antibiotic resistance in an in vitro subgingival biofilm model Oral Microbiol Immunol 2007 ; 22 : 333-339 Liste exhaustive de la bibliographie sur la version digitale de l’article